eye drop manufacturing

Best innovations in Healthcare
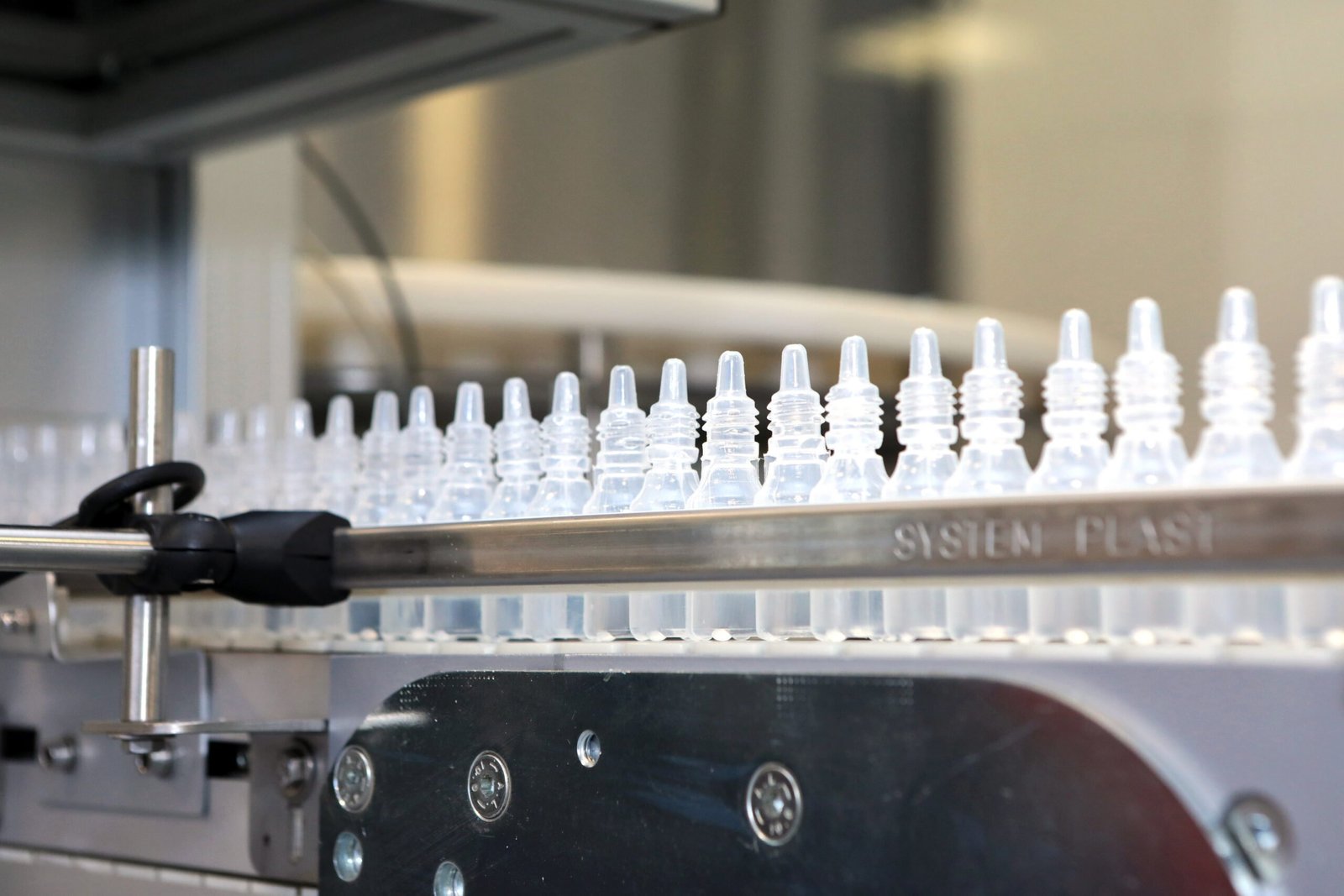
The manufacturing process for eye drops involves several critical steps to ensure the final product is sterile, safe, and effective
Eye drop manufacturing involves the production of sterile liquid solutions designed for application directly into the eyes. These products are essential in the treatment of various ocular conditions, such as allergies, infections, glaucoma, and dry eye syndrome.
The eyes are the window to the soul, and proper care is essential for clarity and health
Features of Project
Demoralized voluptatum deleniti atque corrupti dolores quas molestias excepturi sint occaecati.
Process
Formulation Development
Preparation of Solution
Sterilization
Filling and Sealing
Packaging
Quality Control
Storage and Distribution

At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores quas molestias excepturi sint occaecati cupiditate similique.
Working Process
01
Step
Formulation Development
Active Ingredient Selection: The active pharmaceutical ingredient (API) is chosen based on the therapeutic effect required.
Excipients Selection: Excipients like preservatives, buffers, and viscosity agents are selected to stabilize the formulation and enhance patient comfort.
Sterility and pH Adjustment: The formulation must be adjusted to match the pH of the tear fluid to minimize irritation.
Excipients Selection: Excipients like preservatives, buffers, and viscosity agents are selected to stabilize the formulation and enhance patient comfort.
Sterility and pH Adjustment: The formulation must be adjusted to match the pH of the tear fluid to minimize irritation.
02
Step
Preparation of Solution
Mixing: The active ingredient and excipients are dissolved in purified water. This step is typically done in a controlled environment to prevent contamination.
pH Adjustment: The pH of the solution is carefully adjusted to be compatible with the eye’s natural environment.
Filtration: The solution is passed through filters to remove particulate matter and ensure clarity.
pH Adjustment: The pH of the solution is carefully adjusted to be compatible with the eye’s natural environment.
Filtration: The solution is passed through filters to remove particulate matter and ensure clarity.
03
Step
Sterilization
Autoclaving: The solution may be sterilized using steam under pressure (autoclaving) if the formulation is heat-stable.
Aseptic Filtration: If the formulation is sensitive to heat, it is sterilized using aseptic filtration through a 0.22-micron filter to remove any potential microbial contamination.
Aseptic Filtration: If the formulation is sensitive to heat, it is sterilized using aseptic filtration through a 0.22-micron filter to remove any potential microbial contamination.
04
Step
Filling and Sealing
Aseptic Filling: The sterile solution is filled into sterile containers (bottles or vials) in a sterile environment to prevent contamination.
Sealing: The containers are sealed immediately after filling to maintain sterility. Seals can be screw caps with dropper tips or single-use vials.
Sealing: The containers are sealed immediately after filling to maintain sterility. Seals can be screw caps with dropper tips or single-use vials.
05
Step
Packaging
Labeling: Containers are labeled with necessary information, including the drug name, concentration, expiration date, and storage conditions.
Secondary Packaging: The labeled containers are packed into boxes for protection during transport and storage.
Secondary Packaging: The labeled containers are packed into boxes for protection during transport and storage.
06
Step
Quality Control
Sterility Testing: Samples are tested for sterility to ensure no microbial contamination.
Assay and Content Uniformity: The concentration of the active ingredient is checked to ensure consistency.
pH and Viscosity Testing: The final product is tested to ensure it meets the required specifications for pH and viscosity.
Stability Testing: The product is subjected to stability tests to ensure it remains effective until the expiration date.
Assay and Content Uniformity: The concentration of the active ingredient is checked to ensure consistency.
pH and Viscosity Testing: The final product is tested to ensure it meets the required specifications for pH and viscosity.
Stability Testing: The product is subjected to stability tests to ensure it remains effective until the expiration date.
07
Step
Storage and Distribution
Storage: Eye drops are typically stored in a controlled environment, often at room temperature or refrigerated, depending on the formulation.
Distribution: The final product is distributed to pharmacies, hospitals, or directly to patients.
Distribution: The final product is distributed to pharmacies, hospitals, or directly to patients.
Project Information
Completely synergize resource taxing relationships via premier.
Client Name
John Henry
Category
Metallurgy
Start Time
01 Jan, 2024
End Time
26 Apr, 2024
Budget
$20,00,500
Location
rohini , delhi
