Tablet Manufacturing

BEST INNOVATIONS IN PHARMACEUTICAL MANUFACTURING
Tablet manufacturing and packaging is a key process in the pharmaceutical industry, involving the production of solid dosage forms that are convenient, precise, and stable
Tablet manufacturing and packaging require precise control of formulation, granulation, compression, coating, and packaging processes to produce tablets that meet quality standards and patient expectations. Stringent quality control measures and compliance with regulatory guidelines ensure the production of safe and effective tablets for patients
strategies. Seamlessly visualize quality intellectual capital without superior.”
- John Henry
Features of Project
Demoralized voluptatum deleniti atque corrupti dolores quas molestias excepturi sint occaecati.
At vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores quas molestias excepturi sint occaecati cupiditate similique.
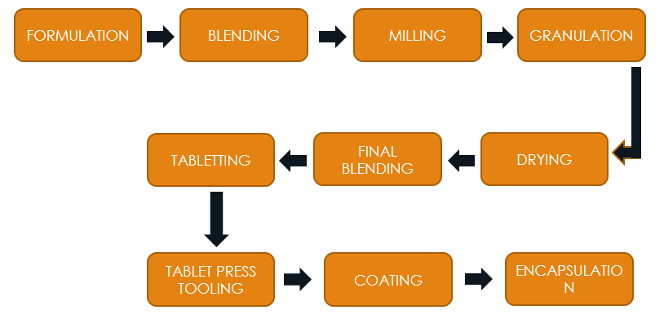
Working Process
Pre-formulation and formulation
Formulation : Blending API with excipients (binders, diluents, disintegrants, lubricants, fillers) to ensure homogeneity and facilitate compression.
Granulation and compression
Compression: Compressing granules into tablets of desired shape, size, and hardness.
Coating (optional)
Quality control
Packaging
Secondary packaging: Placing primary containers in cartons or boxes for additional protection and labeling.
Labeling and serialization
Regulatory compliance and batch release
Project Information
Aspirin
Nonsteroidal Anti-Inflammatory Drug (NSAID): It is commonly used to reduce pain, inflammation, and fever.
